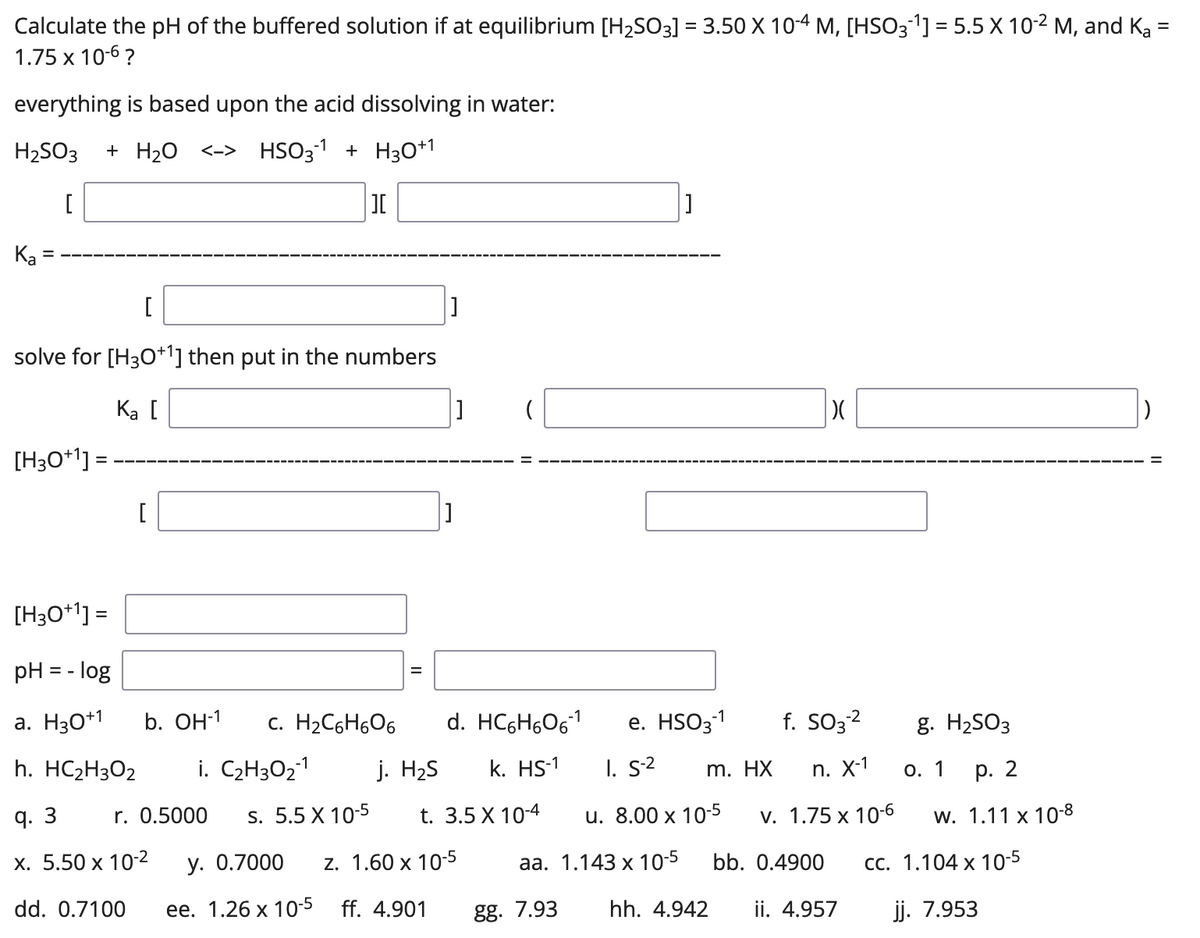
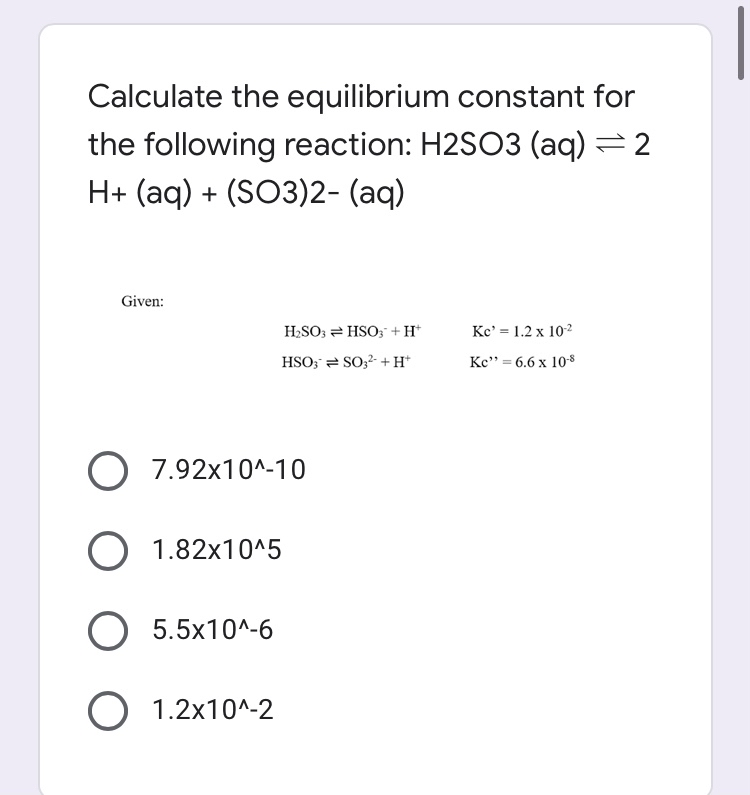
_how-to-balance-so2-h2o-h2so3-sulfur-dioxide-water-preview-hqdefault.jpg)
How to Balance SO2 + H2O = H2SO3 (Sulfur dioxide + Water) from sulphur dioxide formula Watch Video - HiFiMov.co
Sulphurous acid (H2SO3) has Ka1 = 1.7 × 10^–2 and Ka2 = 6.4 × 10^–8. The pH of 0.588 M H2SO3 is ..... - Sarthaks eConnect | Largest Online Education Community

H2SO3 ka lewis structure.how to draw Lewis structure of sulfurous acid.sulfurous acid.class 11-12 - YouTube

Which expression represents the equilibrium constant expression for sulphurous acid (H2SO3)? - Brainly.com
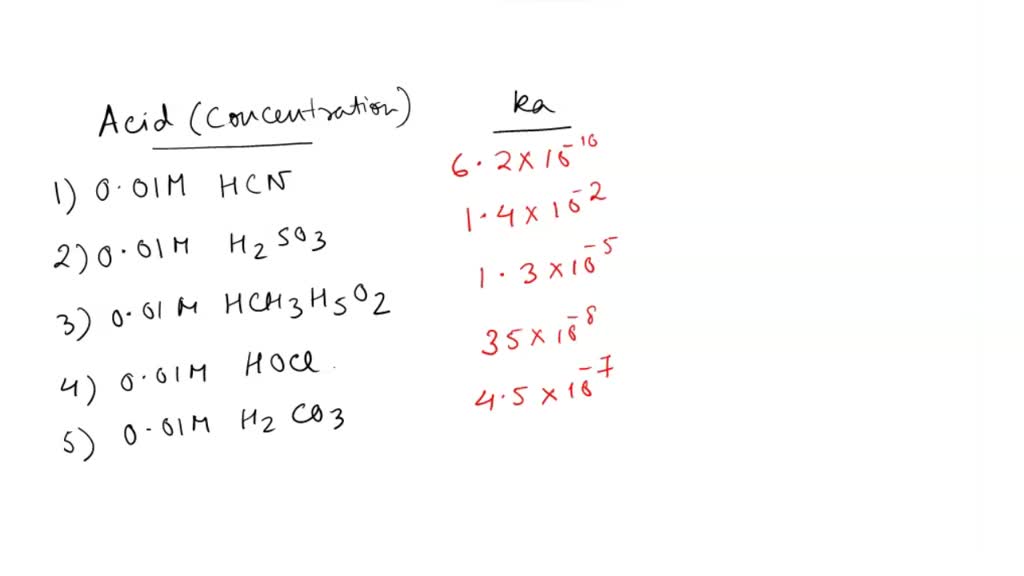
SOLVED: 'QUESTION 3 In which of the following aqueous solutions does the weak acid exhibit the highest percentage ionization? 0.01 M HCN (Ka = 6.2 x 10-10) 0.01 M H2SO3 (Ka =
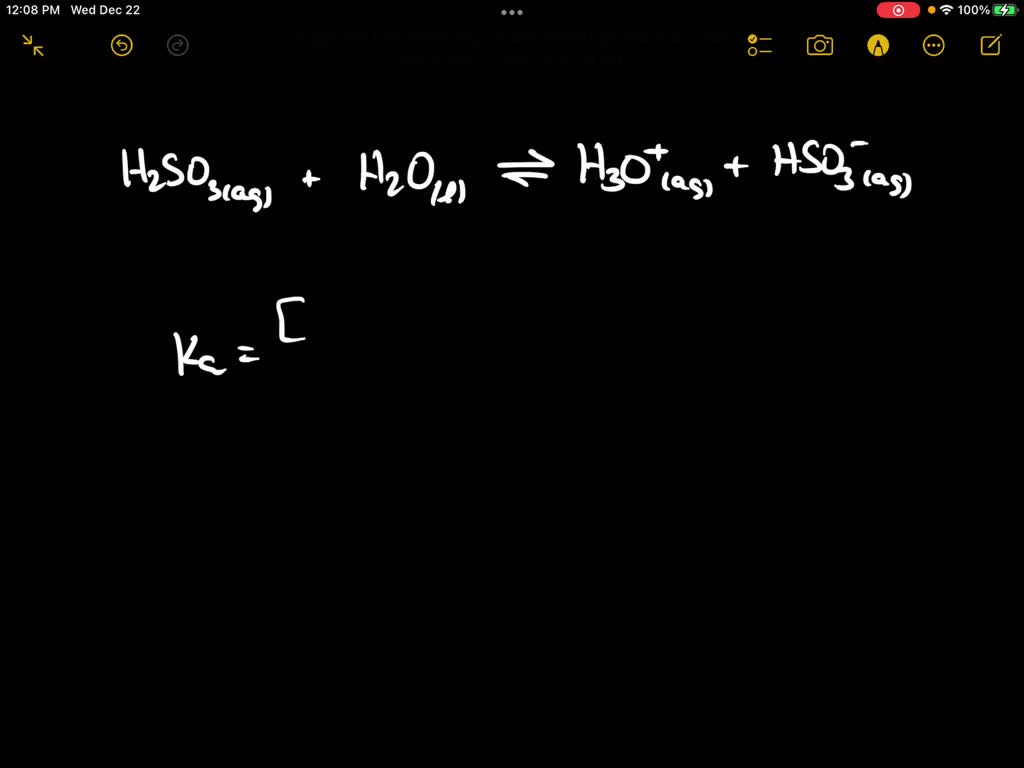



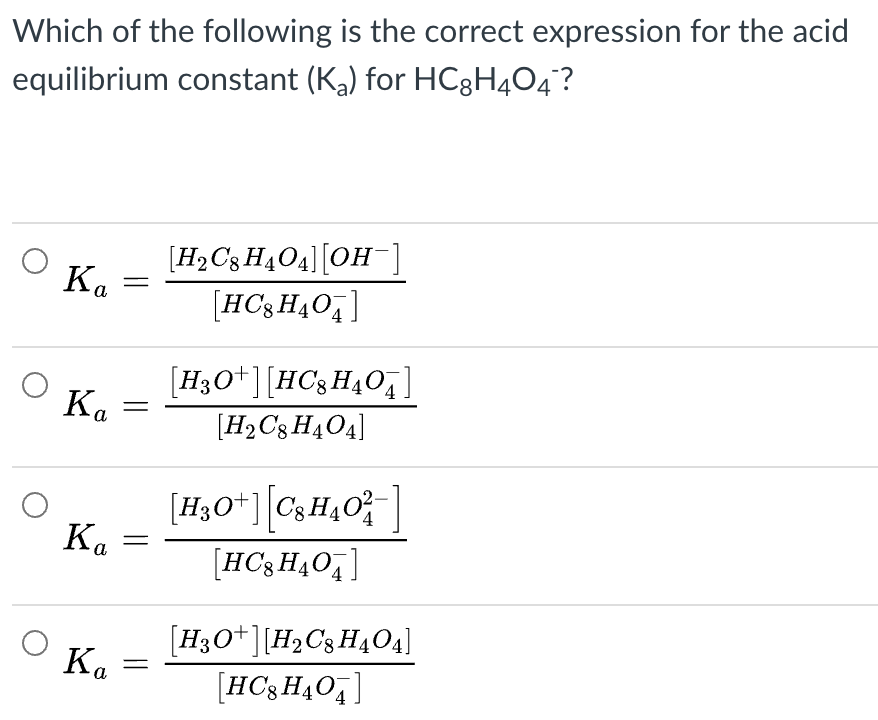
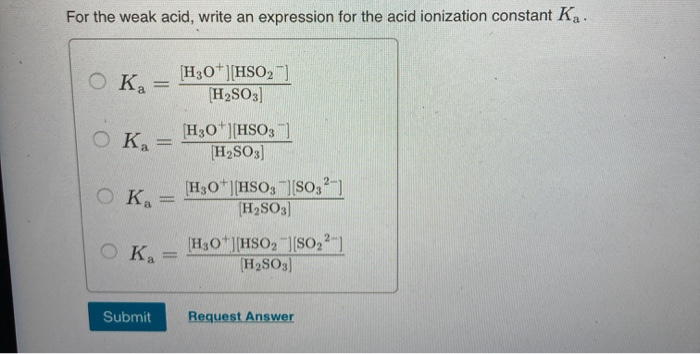



![Solved Question 11 (4 points) The expression: [H+][HSO3-] / | Chegg.com Solved Question 11 (4 points) The expression: [H+][HSO3-] / | Chegg.com](https://media.cheggcdn.com/media/117/117b35d3-7ad8-4855-bfc6-0e3f43ac0e19/phpwEDxY2.png)